Which Eas Reaction Can Be Used to Make a Carbon-carbon Bond in the Synthesis of Diazepam
Chapter 20. Organic Chemistry
twenty.1 Hydrocarbons
Learning Objectives
By the stop of this section, you will be able to:
- Explain the importance of hydrocarbons and the reason for their diversity
- Name saturated and unsaturated hydrocarbons, and molecules derived from them
- Draw the reactions characteristic of saturated and unsaturated hydrocarbons
- Identify structural and geometric isomers of hydrocarbons
The largest database[i] of organic compounds lists nearly ten million substances, which include compounds originating from living organisms and those synthesized by chemists. The number of potential organic compounds has been estimated[2] at x60—an astronomically high number. The being of and so many organic molecules is a upshot of the ability of carbon atoms to grade upwards to four strong bonds to other carbon atoms, resulting in chains and rings of many different sizes, shapes, and complexities.
The simplest organic compounds incorporate only the elements carbon and hydrogen, and are called hydrocarbons. Even though they are equanimous of only 2 types of atoms, there is a wide variety of hydrocarbons because they may consist of varying lengths of bondage, branched chains, and rings of carbon atoms, or combinations of these structures. In improver, hydrocarbons may differ in the types of carbon-carbon bonds nowadays in their molecules. Many hydrocarbons are establish in plants, animals, and their fossils; other hydrocarbons accept been prepared in the laboratory. We use hydrocarbons every day, mainly every bit fuels, such equally natural gas, acetylene, propane, butane, and the principal components of gasoline, diesel fuel, and heating oil. The familiar plastics polyethylene, polypropylene, and polystyrene are also hydrocarbons. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. This leads to differences in geometries and in the hybridization of the carbon orbitals.
Alkanes
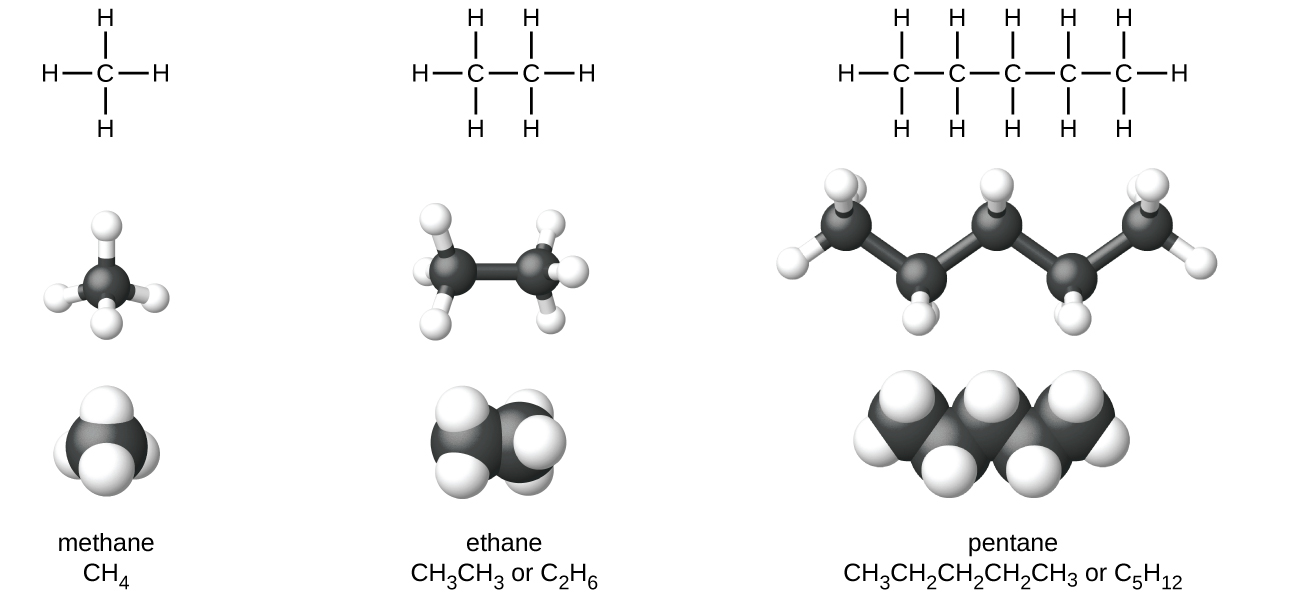
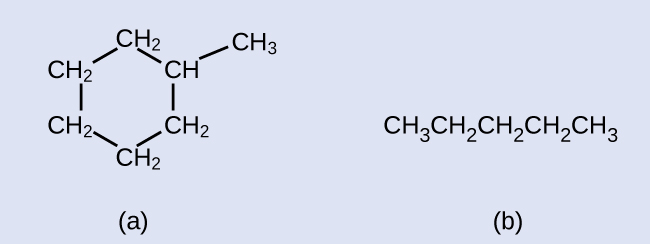
Alkanes, or saturated hydrocarbons, contain only single covalent bonds between carbon atoms. Each of the carbon atoms in an methane series has sp iii hybrid orbitals and is bonded to iv other atoms, each of which is either carbon or hydrogen. The Lewis structures and models of methyl hydride, ethane, and pentane are illustrated in Figure 1. Carbon chains are usually drawn as straight lines in Lewis structures, but one has to remember that Lewis structures are not intended to point the geometry of molecules. Detect that the carbon atoms in the structural models (the ball-and-stick and space-filling models) of the pentane molecule do not prevarication in a straight line. Because of the sp three hybridization, the bond angles in carbon chains are shut to 109.5°, giving such bondage in an paraffin a zigzag shape.
The structures of alkanes and other organic molecules may also be represented in a less detailed manner by condensed structural formulas (or only, condensed formulas). Instead of the usual format for chemical formulas in which each chemical element symbol appears just one time, a condensed formula is written to suggest the bonding in the molecule. These formulas have the appearance of a Lewis structure from which most or all of the bond symbols take been removed. Condensed structural formulas for ethane and pentane are shown at the bottom of Figure one, and several additional examples are provided in the exercises at the end of this affiliate.
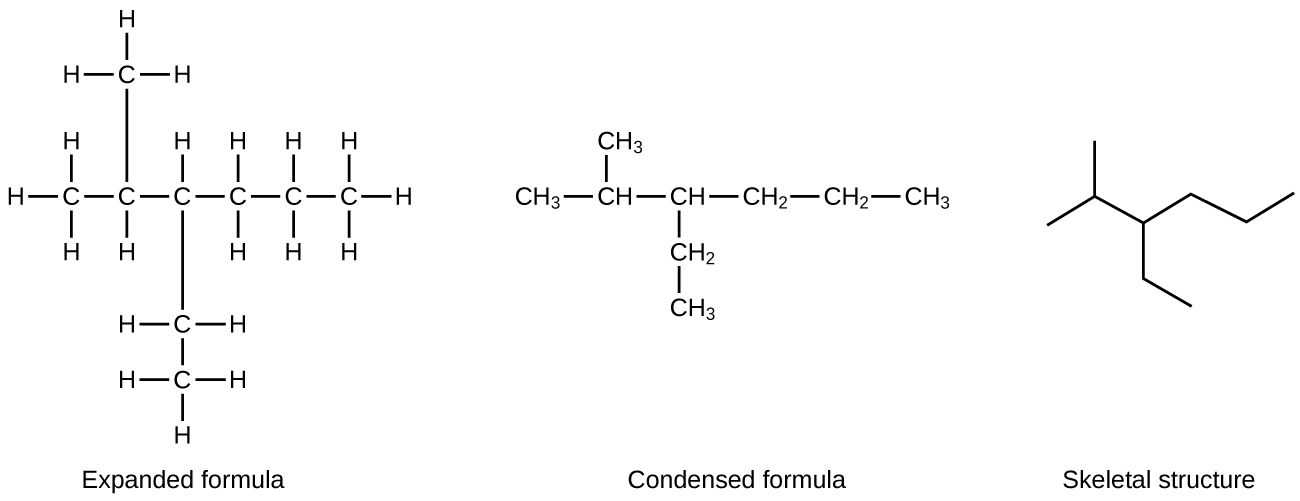
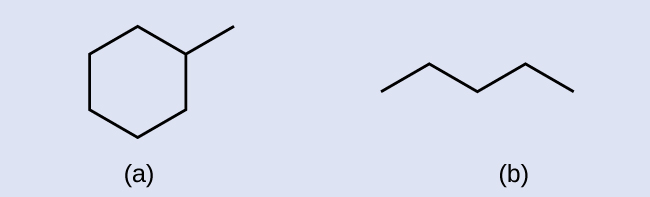
A common method used by organic chemists to simplify the drawings of larger molecules is to utilize a skeletal structure (too called a line-angle structure). In this type of structure, carbon atoms are not symbolized with a C, but represented past each end of a line or bend in a line. Hydrogen atoms are not drawn if they are fastened to a carbon. Other atoms as well carbon and hydrogen are represented by their elemental symbols. Figure 2 shows 3 different ways to describe the same construction.
Example 1
Drawing Skeletal Structures
Draw the skeletal structures for these 2 molecules:
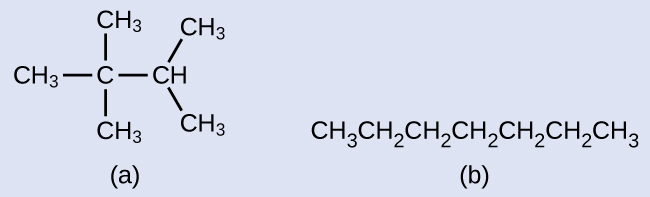
Solution
Each carbon atom is converted into the terminate of a line or the place where lines intersect. All hydrogen atoms attached to the carbon atoms are left out of the construction (although we yet demand to recognize they are there):
Cheque Your Learning
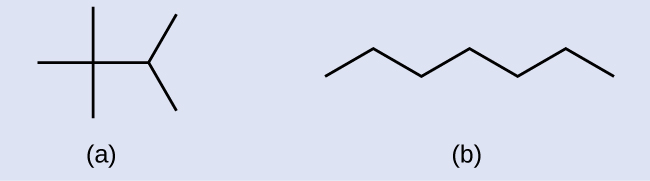
Draw the skeletal structures for these ii molecules:
Answer:
Instance two
Interpreting Skeletal Structures
Identify the chemical formula of the molecule represented hither:
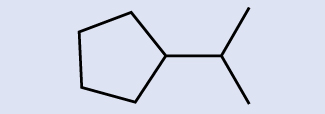
Solution
There are eight places where lines intersect or end, pregnant that there are 8 carbon atoms in the molecule. Since we know that carbon atoms tend to make four bonds, each carbon cantlet will take the number of hydrogen atoms that are required for four bonds. This compound contains 16 hydrogen atoms for a molecular formula of CviiiH16.
Location of the hydrogen atoms:
Check Your Learning
Identify the chemical formula of the molecule represented hither:
All alkanes are composed of carbon and hydrogen atoms, and have similar bonds, structures, and formulas; noncyclic alkanes all have a formula of CnorthwardH2n+2. The number of carbon atoms present in an methane series has no limit. Greater numbers of atoms in the molecules volition pb to stronger intermolecular attractions (dispersion forces) and correspondingly different physical properties of the molecules. Properties such as melting point and boiling signal (Tabular array one) ordinarily change smoothly and predictably equally the number of carbon and hydrogen atoms in the molecules change.
| Alkane series | Molecular Formula | Melting Point (°C) | Humid Point (°C) | Stage at STP[3] | Number of Structural Isomers |
|---|---|---|---|---|---|
| methane | CH4 | –182.v | –161.5 | gas | 1 |
| ethane | CtwoH6 | –183.iii | –88.vi | gas | 1 |
| propane | C3H8 | –187.seven | –42.i | gas | one |
| butane | CfourH10 | –138.3 | –0.5 | gas | ii |
| pentane | C5H12 | –129.vii | 36.ane | liquid | iii |
| hexane | CsixH14 | –95.3 | 68.vii | liquid | 5 |
| heptane | C7H16 | –xc.6 | 98.4 | liquid | 9 |
| octane | CviiiH18 | –56.eight | 125.7 | liquid | 18 |
| nonane | C9Hxx | –53.six | 150.viii | liquid | 35 |
| decane | CtenH22 | –29.7 | 174.0 | liquid | 75 |
| tetradecane | CfourteenHxxx | 5.ix | 253.5 | solid | 1858 |
| octadecane | CeighteenH38 | 28.2 | 316.i | solid | 60,523 |
| Table 1. Backdrop of Some Alkanes[4] | |||||
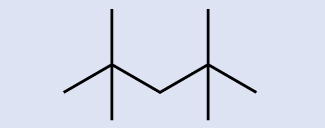
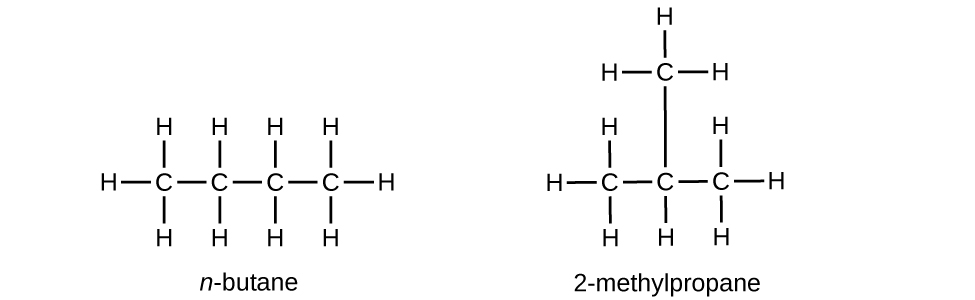
Hydrocarbons with the aforementioned formula, including alkanes, tin have unlike structures. For example, ii alkanes have the formula C4Hten: They are called n-butane and 2-methylpropane (or isobutane), and have the following Lewis structures:
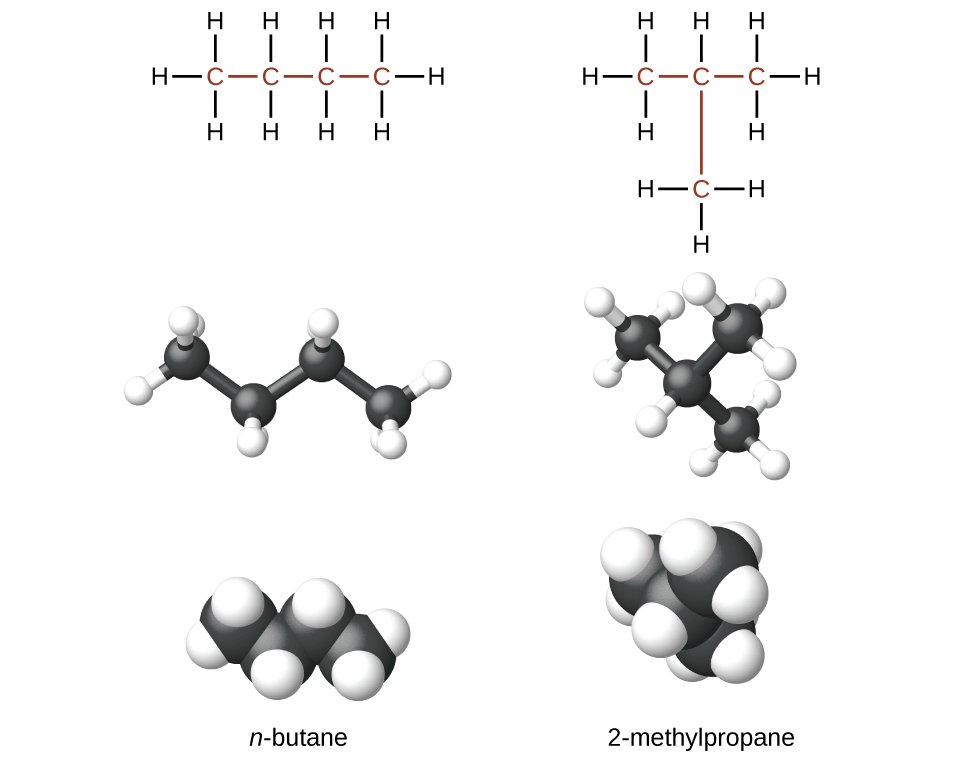
The compounds n-butane and 2-methylpropane are structural isomers (the term constitutional isomers is also commonly used). Constitutional isomers accept the same molecular formula but different spatial arrangements of the atoms in their molecules. The n-butane molecule contains an unbranched chain, meaning that no carbon atom is bonded to more than than two other carbon atoms. We use the term normal, or the prefix n, to refer to a chain of carbon atoms without branching. The compound 2–methylpropane has a branched chain (the carbon atom in the middle of the Lewis structure is bonded to 3 other carbon atoms)
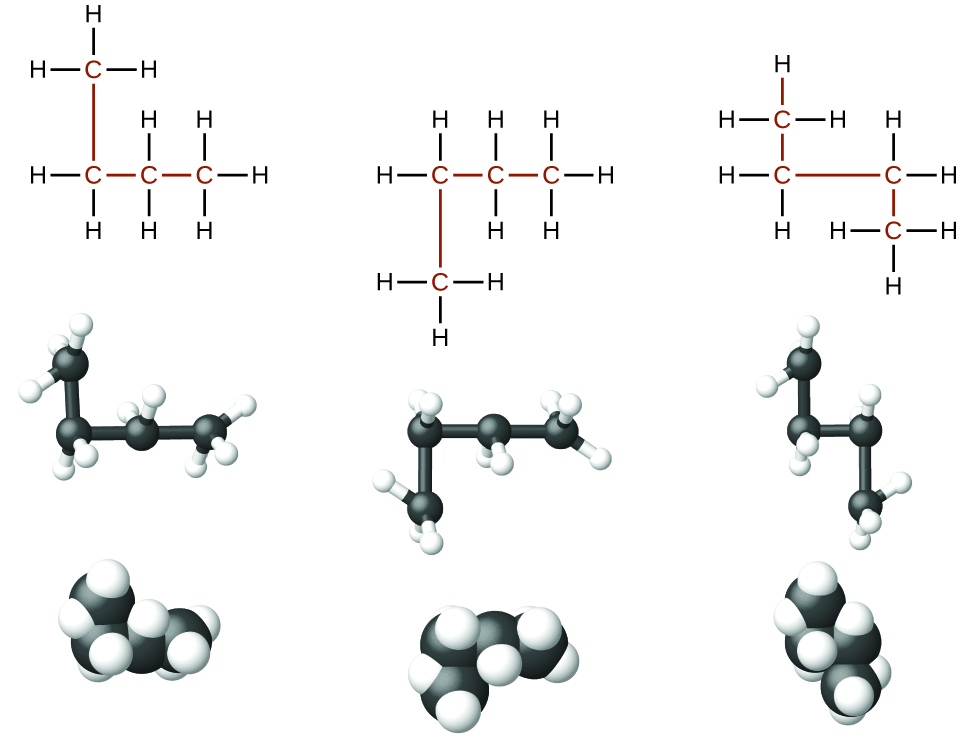
Identifying isomers from Lewis structures is not equally like shooting fish in a barrel as it looks. Lewis structures that look different may actually represent the same isomers. For example, the three structures in Figure three all represent the aforementioned molecule, n-butane, and hence are not unlike isomers. They are identical because each contains an unbranched chain of four carbon atoms.
The Basics of Organic Nomenclature: Naming Alkanes
The International Spousal relationship of Pure and Applied Chemistry (IUPAC) has devised a system of nomenclature that begins with the names of the alkanes and can exist adjusted from in that location to account for more than complicated structures. The classification for alkanes is based on two rules:
- To proper noun an alkane, first place the longest concatenation of carbon atoms in its structure. A two-carbon chain is called ethane; a three-carbon chain, propane; and a iv-carbon concatenation, butane. Longer bondage are named equally follows: pentane (five-carbon concatenation), hexane (six), heptane (7), octane (8), nonane (9), and decane (ten). These prefixes can be seen in the names of the alkanes described in Table 1.
- Add prefixes to the name of the longest concatenation to indicate the positions and names of substituents. Substituents are branches or functional groups that replace hydrogen atoms on a concatenation. The position of a substituent or branch is identified past the number of the carbon cantlet it is bonded to in the chain. Nosotros number the carbon atoms in the chain by counting from the end of the concatenation nearest the substituents. Multiple substituents are named individually and placed in alphabetical order at the front end of the name.
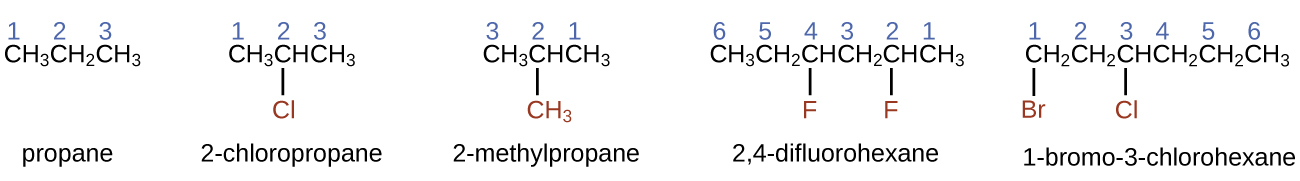
When more than than one substituent is nowadays, either on the same carbon atom or on different carbon atoms, the substituents are listed alphabetically. Considering the carbon cantlet numbering begins at the end closest to a substituent, the longest chain of carbon atoms is numbered in such a fashion as to produce the lowest number for the substituents. The ending -o replaces -ide at the stop of the name of an electronegative substituent (in ionic compounds, the negatively charged ion ends with -ide like chloride; in organic compounds, such atoms are treated as substituents and the -o ending is used). The number of substituents of the same type is indicated by the prefixes di- (two), tri- (three), tetra- (four), and then on (for example, difluoro- indicates 2 fluoride substituents).
Instance iii
Naming Halogen-substituted Alkanes
Name the molecule whose structure is shown here:
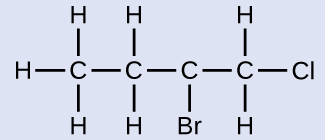
Solution
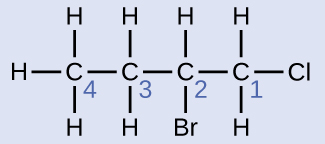
The four-carbon chain is numbered from the end with the chlorine cantlet. This puts the substituents on positions 1 and 2 (numbering from the other end would put the substituents on positions three and 4). Four carbon atoms means that the base of operations name of this compound will be butane. The bromine at position 2 will be described past calculation 2-bromo-; this will come up at the first of the name, since bromo- comes earlier chloro- alphabetically. The chlorine at position one will be described by adding 1-chloro-, resulting in the name of the molecule being two-bromo-1-chlorobutane.
Check Your Learning
Proper name the following molecule:
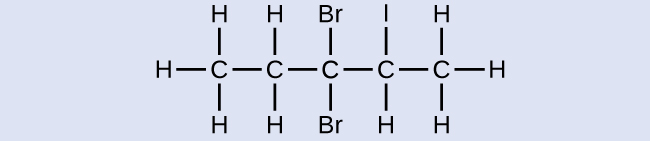
Reply:
3,3-dibromo-2-iodopentane
We phone call a substituent that contains one less hydrogen than the respective methane series an alkyl group. The name of an alkyl group is obtained by dropping the suffix -ane of the alkane series name and adding -yl:
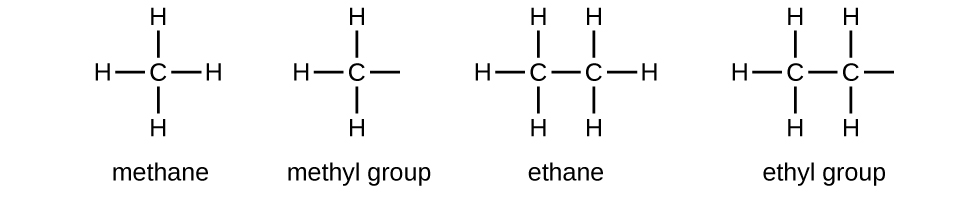
The open bonds in the methyl and ethyl groups point that these alkyl groups are bonded to another atom.
Example 4
Naming Substituted Alkanes
Proper name the molecule whose structure is shown here:
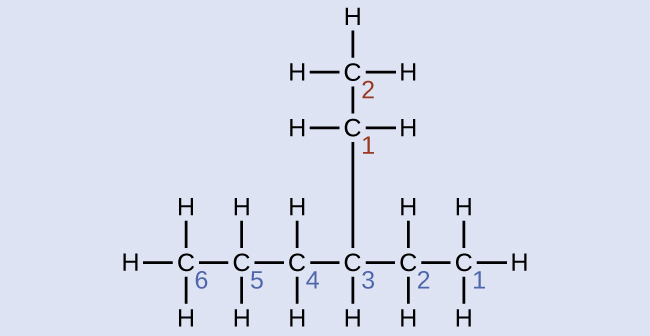
Solution
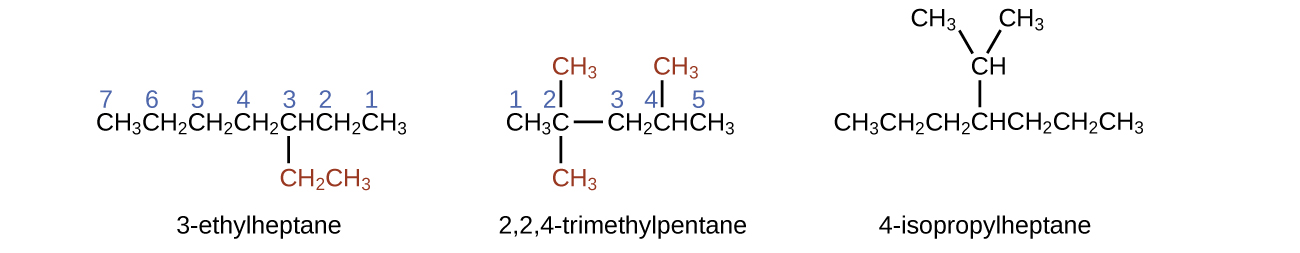
The longest carbon chain runs horizontally across the folio and contains six carbon atoms (this makes the base of the name hexane, but we will also need to incorporate the name of the branch). In this example, we desire to number from right to left (as shown by the bluish numbers) so the branch is continued to carbon three (imagine the numbers from left to right—this would put the co-operative on carbon four, violating our rules). The branch attached to position 3 of our chain contains two carbon atoms (numbered in red)—so we take our proper name for ii carbons eth- and attach -yl at the end to signify we are describing a co-operative. Putting all the pieces together, this molecule is three-ethylhexane.
Cheque Your Learning
Name the following molecule:
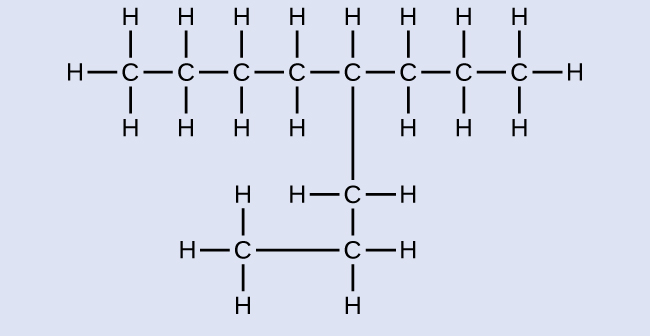
Some hydrocarbons can form more than one type of alkyl group when the hydrogen atoms that would be removed have different "environments" in the molecule. This diversity of possible alkyl groups tin can be identified in the following way: The iv hydrogen atoms in a methyl hydride molecule are equivalent; they all have the same surroundings. They are equivalent considering each is bonded to a carbon atom (the same carbon atom) that is bonded to iii hydrogen atoms. (It may be easier to see the equivalency in the brawl and stick models in Figure ane. Removal of whatsoever one of the four hydrogen atoms from methyl hydride forms a methyl group. Also, the half-dozen hydrogen atoms in ethane are equivalent (Figure 1) and removing any i of these hydrogen atoms produces an ethyl group. Each of the half dozen hydrogen atoms is bonded to a carbon atom that is bonded to two other hydrogen atoms and a carbon atom. However, in both propane and two–methylpropane, there are hydrogen atoms in ii dissimilar environments, distinguished by the next atoms or groups of atoms:
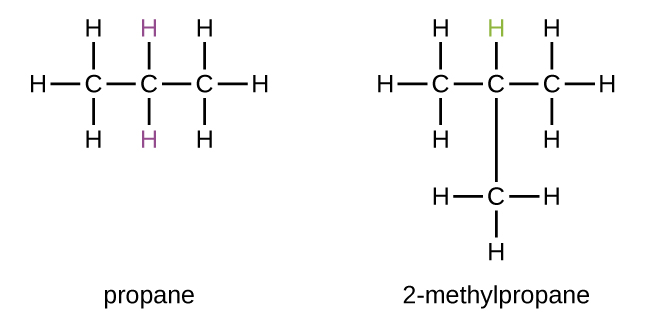
Each of the six equivalent hydrogen atoms of the kickoff blazon in propane and each of the nine equivalent hydrogen atoms of that blazon in 2-methylpropane (all shown in black) are bonded to a carbon atom that is bonded to merely i other carbon atom. The two purple hydrogen atoms in propane are of a 2d type. They differ from the 6 hydrogen atoms of the first type in that they are bonded to a carbon atom bonded to two other carbon atoms. The dark-green hydrogen atom in two-methylpropane differs from the other ix hydrogen atoms in that molecule and from the regal hydrogen atoms in propane. The green hydrogen atom in 2-methylpropane is bonded to a carbon atom bonded to three other carbon atoms. 2 different alkyl groups can be formed from each of these molecules, depending on which hydrogen cantlet is removed. The names and structures of these and several other alkyl groups are listed in Figure 4.
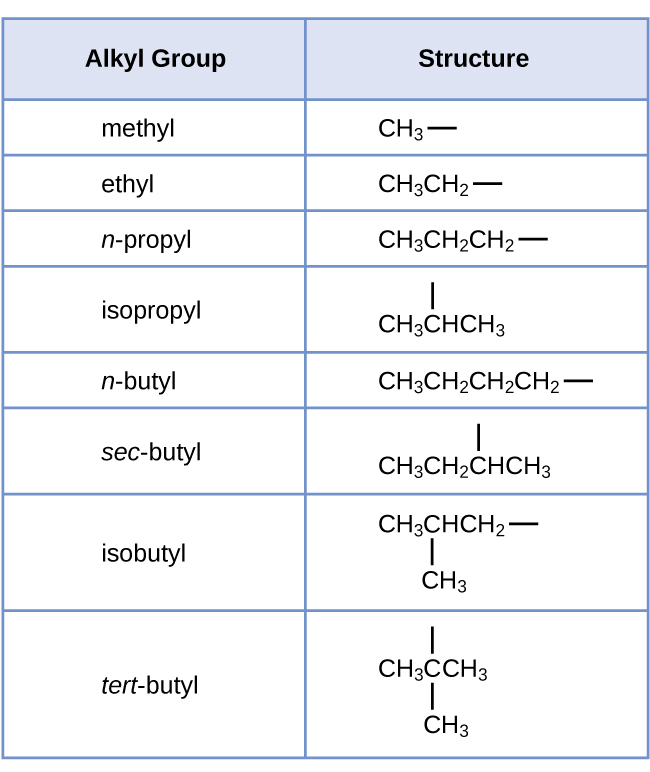
Note that alkyl groups do non exist equally stable independent entities. They are e'er a role of some larger molecule. The location of an alkyl group on a hydrocarbon concatenation is indicated in the same way as any other substituent:
Alkanes are relatively stable molecules, but oestrus or light will activate reactions that involve the breaking of C–H or C–C single bonds. Combustion is one such reaction:
[latex]\text{CH}_4(g)\;+\;2\text{O}_2(g)\;{\longrightarrow}\;\text{CO}_2(g)\;+\;two\text{H}_2\text{O}(g)[/latex]
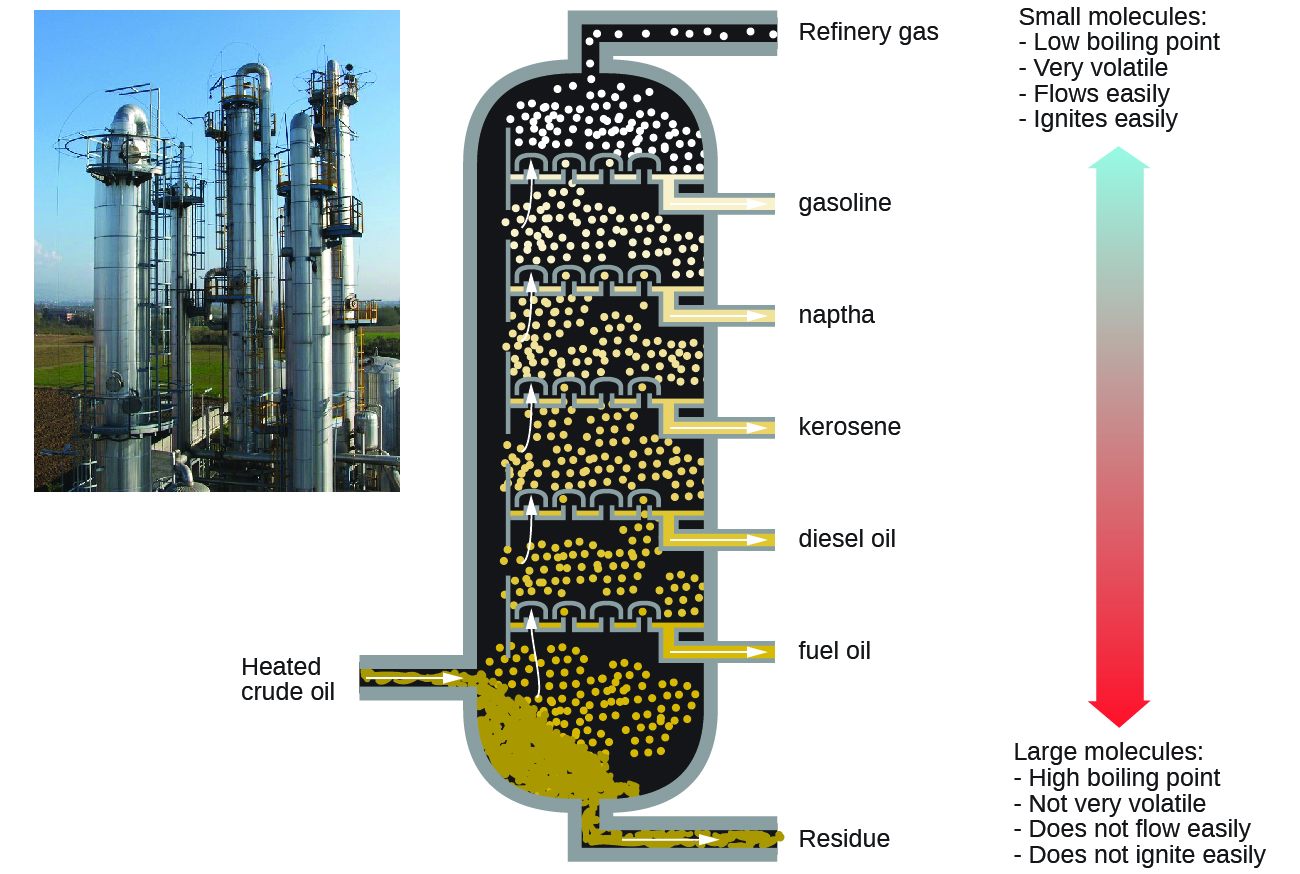
Alkanes burn in the presence of oxygen, a highly exothermic oxidation-reduction reaction that produces carbon dioxide and water. Equally a result, alkanes are fantabulous fuels. For case, methane, CH4, is the principal component of natural gas. Butane, C4Hx, used in camping stoves and lighters is an alkane. Gasoline is a liquid mixture of continuous- and branched-concatenation alkanes, each containing from five to 9 carbon atoms, plus various additives to improve its performance as a fuel. Kerosene, diesel oil, and fuel oil are primarily mixtures of alkanes with college molecular masses. The main source of these liquid alkane fuels is crude oil, a circuitous mixture that is separated by fractional distillation. Partial distillation takes advantage of differences in the boiling points of the components of the mixture (see Figure 5). You may recall that boiling point is a part of intermolecular interactions, which was discussed in the chapter on solutions and colloids.
In a exchange reaction, another typical reaction of alkanes, one or more of the methane series'south hydrogen atoms is replaced with a different atom or group of atoms. No carbon-carbon bonds are cleaved in these reactions, and the hybridization of the carbon atoms does not change. For example, the reaction between ethane and molecular chlorine depicted here is a substitution reaction:
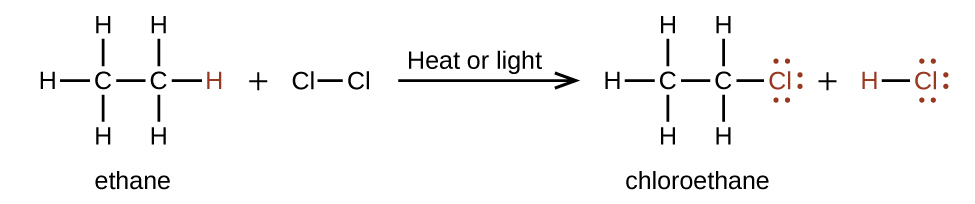
The C–Cl portion of the chloroethane molecule is an example of a functional grouping, the role or moiety of a molecule that imparts a specific chemical reactivity. The types of functional groups present in an organic molecule are major determinants of its chemical properties and are used as a means of classifying organic compounds as detailed in the remaining sections of this affiliate.

Want more exercise naming alkanes? Sentinel this cursory video tutorial to review the nomenclature procedure.
Alkenes
Organic compounds that contain 1 or more double or triple bonds between carbon atoms are described as unsaturated. You take probable heard of unsaturated fats. These are circuitous organic molecules with long bondage of carbon atoms, which contain at to the lowest degree one double bond betwixt carbon atoms. Unsaturated hydrocarbon molecules that incorporate one or more than double bonds are called alkenes. Carbon atoms linked by a double bond are jump together past 2 bonds, one σ bond and one π bond. Double and triple bonds requite rise to a different geometry around the carbon atom that participates in them, leading to important differences in molecular shape and properties. The differing geometries are responsible for the dissimilar properties of unsaturated versus saturated fats.
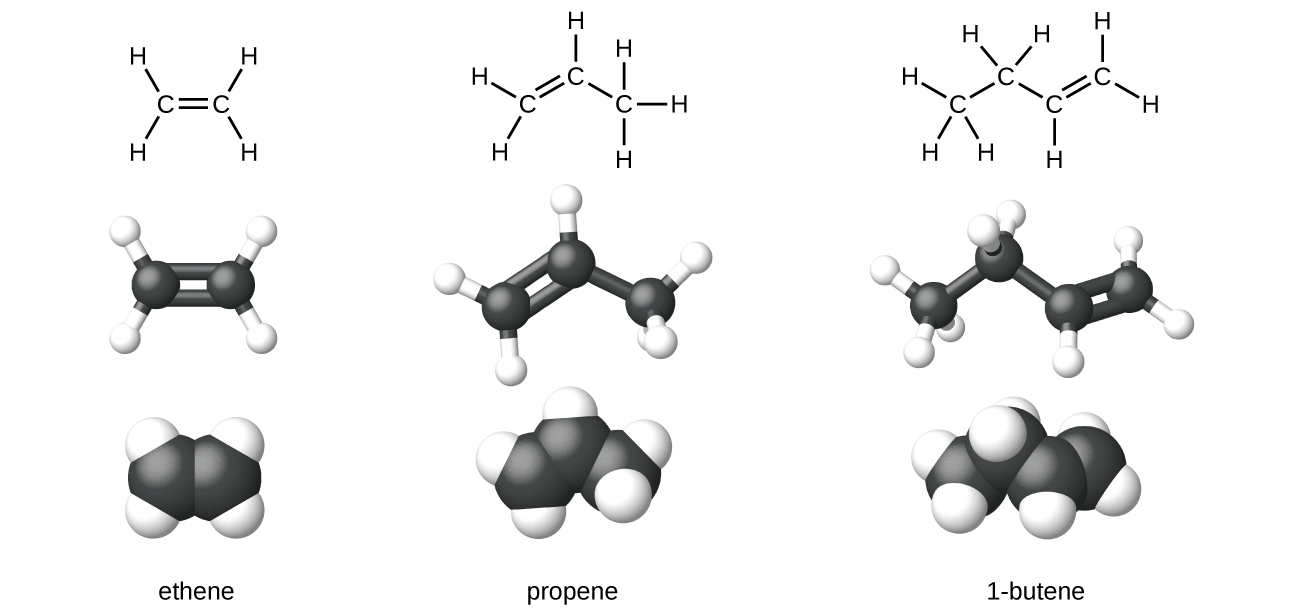
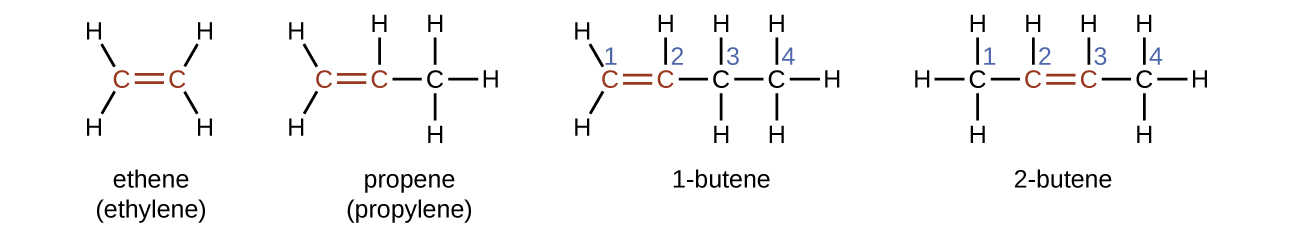
Ethene, C2H4, is the simplest alkene. Each carbon cantlet in ethene, commonly called ethylene, has a trigonal planar structure. The second member of the series is propene (propylene) (Figure 6); the butene isomers follow in the serial. Four carbon atoms in the chain of butene allows for the formation of isomers based on the position of the double bond, equally well as a new grade of isomerism.
Ethylene (the common industrial name for ethene) is a basic raw material in the production of polyethylene and other important compounds. Over 135 meg tons of ethylene were produced worldwide in 2010 for employ in the polymer, petrochemical, and plastic industries. Ethylene is produced industrially in a procedure chosen cracking, in which the long hydrocarbon chains in a petroleum mixture are broken into smaller molecules.
Recycling Plastics
Polymers (from Greek words poly pregnant "many" and mer meaning "parts") are large molecules made upwardly of repeating units, referred to every bit monomers. Polymers can be natural (starch is a polymer of sugar residues and proteins are polymers of amino acids) or synthetic [like polyethylene, polyvinyl chloride (PVC), and polystyrene]. The diversity of structures of polymers translates into a broad range of properties and uses that make them integral parts of our everyday lives. Calculation functional groups to the construction of a polymer tin result in significantly different properties (see the discussion about Kevlar later in this affiliate).
An example of a polymerization reaction is shown in Effigy 7. The monomer ethylene (CiiHiv) is a gas at room temperature, but when polymerized, using a transition metal catalyst, it is transformed into a solid material made upwardly of long bondage of –CH2– units called polyethylene. Polyethylene is a article plastic used primarily for packaging (bags and films).
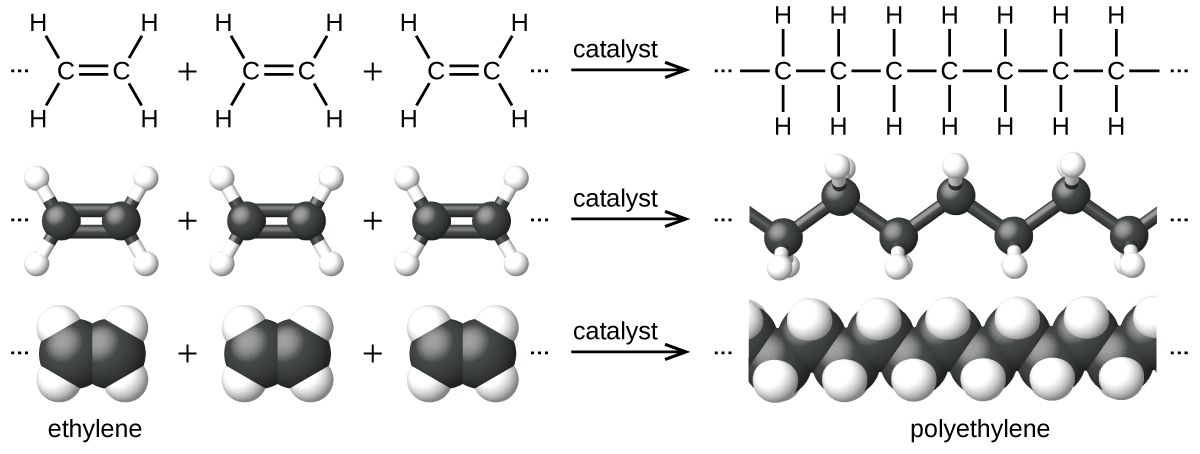
Polyethylene is a member of one subset of synthetic polymers classified every bit plastics. Plastics are synthetic organic solids that tin can be molded; they are typically organic polymers with loftier molecular masses. Almost of the monomers that get into common plastics (ethylene, propylene, vinyl chloride, styrene, and ethylene terephthalate) are derived from petrochemicals and are not very biodegradable, making them candidate materials for recycling. Recycling plastics helps minimize the demand for using more of the petrochemical supplies and as well minimizes the environmental damage caused by throwing away these nonbiodegradable materials.
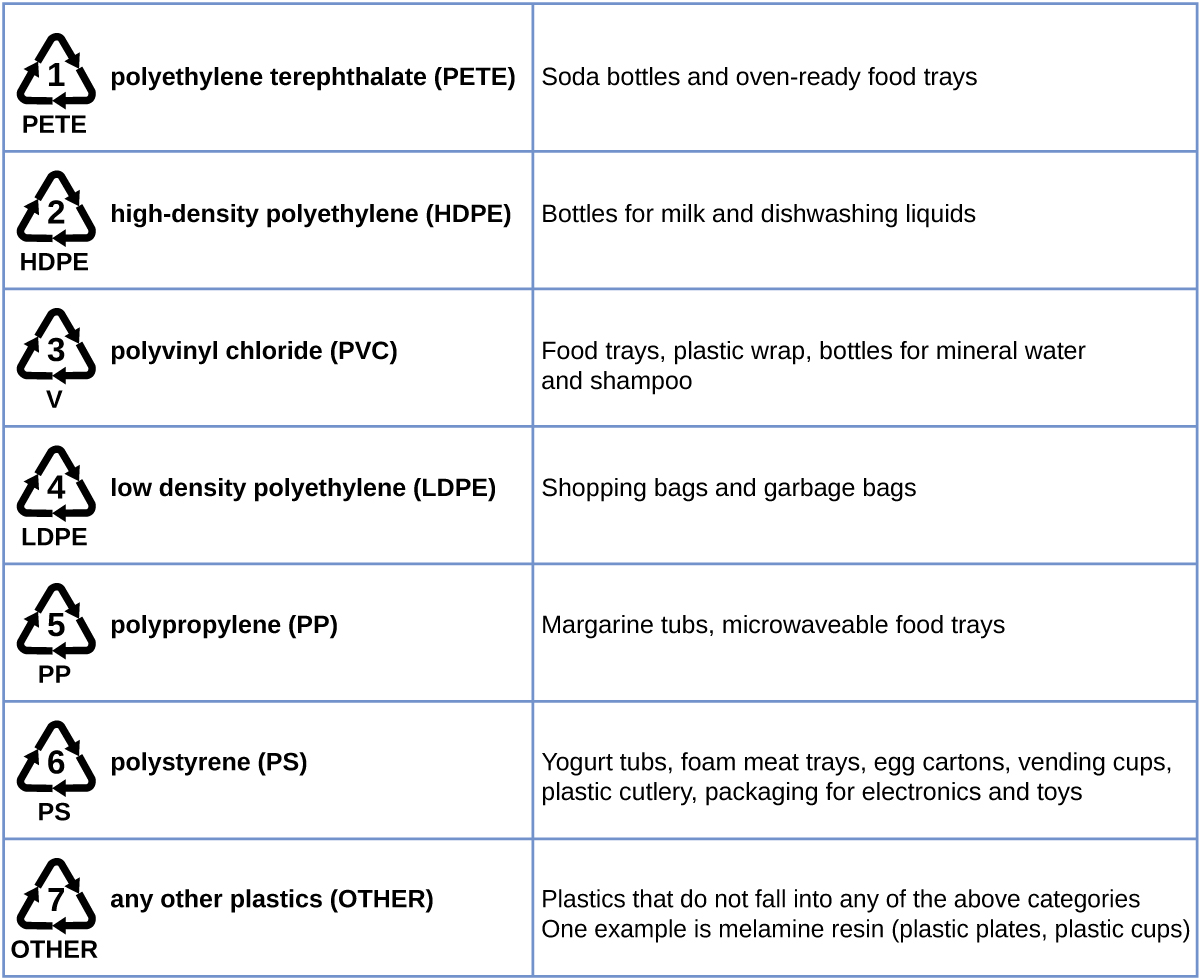
Plastic recycling is the procedure of recovering waste, scrap, or used plastics, and reprocessing the material into useful products. For case, polyethylene terephthalate (soft beverage bottles) can be melted downward and used for plastic furniture, in carpets, or for other applications. Other plastics, like polyethylene (bags) and polypropylene (cups, plastic food containers), can exist recycled or reprocessed to exist used again. Many areas of the country have recycling programs that focus on 1 or more of the article plastics that take been assigned a recycling code (see Effigy viii). These operations accept been in effect since the 1970s and take made the product of some plastics among the about efficient industrial operations today.
The name of an alkene is derived from the name of the paraffin with the same number of carbon atoms. The presence of the double bond is signified by replacing the suffix -ane with the suffix -ene. The location of the double bond is identified past naming the smaller of the numbers of the carbon atoms participating in the double bond:
Isomers of Alkenes
Molecules of 1-butene and 2-butene are structural isomers; the organisation of the atoms in these two molecules differs. As an instance of arrangement differences, the first carbon atom in i-butene is bonded to ii hydrogen atoms; the first carbon atom in 2-butene is bonded to three hydrogen atoms.
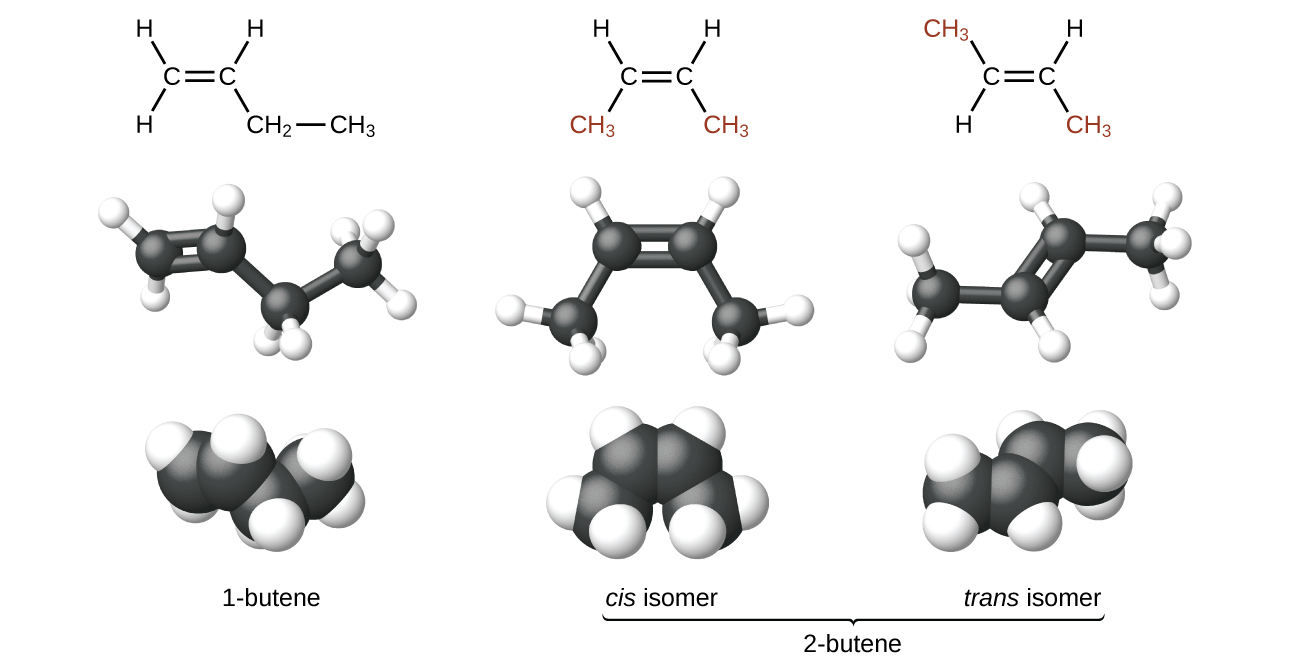
The compound 2-butene and some other alkenes likewise form a second type of isomer called a geometric isomer. In a set of geometric isomers, the same types of atoms are attached to each other in the same order, simply the geometries of the two molecules differ. Geometric isomers of alkenes differ in the orientation of the groups on either side of a [latex]\text{C}\;=\;\text{C}[/latex] bond.
Carbon atoms are costless to rotate around a single bond but not effectually a double bail; a double bail is rigid. This makes it possible to take two isomers of 2-butene, 1 with both methyl groups on the same side of the double bond and 1 with the methyl groups on reverse sides. When structures of butene are drawn with 120° bail angles around the sp two-hybridized carbon atoms participating in the double bond, the isomers are apparent. The 2-butene isomer in which the ii methyl groups are on the same side is called a cis-isomer; the one in which the two methyl groups are on opposite sides is chosen a trans-isomer (Figure 9). The different geometries produce different physical properties, such as boiling point, that may make separation of the isomers possible:
Alkenes are much more reactive than alkanes because the [latex]\text{C}\;=\;\text{C}[/latex] moiety is a reactive functional grouping. A π bond, beingness a weaker bond, is disrupted much more than easily than a σ bond. Thus, alkenes undergo a characteristic reaction in which the π bail is cleaved and replaced by two σ bonds. This reaction is called an addition reaction. The hybridization of the carbon atoms in the double bond in an alkene changes from sp ii to sp 3 during an addition reaction. For example, halogens add to the double bond in an alkene instead of replacing hydrogen, as occurs in an alkane series:
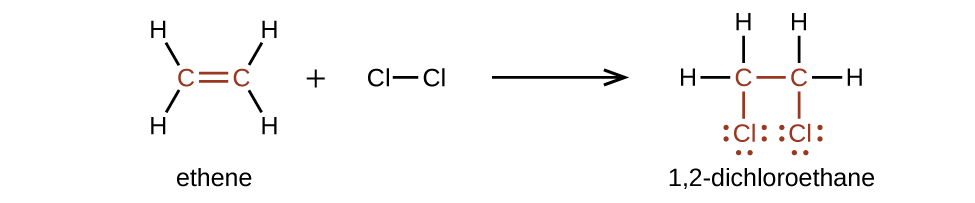
Example 5
Alkene Reactivity and Naming
Provide the IUPAC names for the reactant and product of the halogenation reaction shown here:
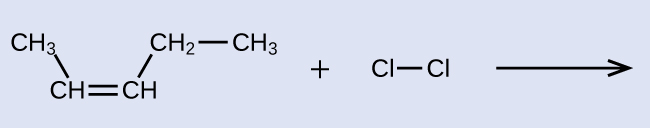
Solution
The reactant is a five-carbon chain that contains a carbon-carbon double bail, then the base name will exist pentene. We begin counting at the end of the chain closest to the double bond—in this example, from the left—the double bail spans carbons ii and 3, then the proper name becomes 2-pentene. Since there are two carbon-containing groups attached to the 2 carbon atoms in the double bond—and they are on the same side of the double bail—this molecule is the cis-isomer, making the name of the starting alkene cis-two-pentene. The product of the halogenation reaction will take two chlorine atoms attached to the carbon atoms that were a function of the carbon-carbon double bond:
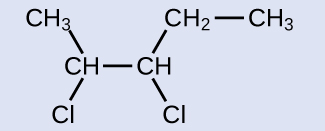
This molecule is now a substituted alkane and will exist named as such. The base of the proper noun will exist pentane. Nosotros will count from the end that numbers the carbon atoms where the chlorine atoms are attached as 2 and 3, making the name of the product two,3-dichloropentane.
Cheque Your Learning
Provide names for the reactant and production of the reaction shown:
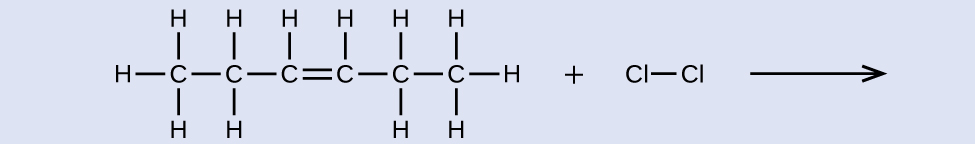
Answer:
reactant: cis-3-hexene product: 3,4-dichlorohexane
Alkynes
Hydrocarbon molecules with 1 or more triple bonds are chosen alkynes; they make up another serial of unsaturated hydrocarbons. Two carbon atoms joined by a triple bond are bound together past one σ bond and two π bonds. The sp-hybridized carbons involved in the triple bond accept bond angles of 180°, giving these types of bonds a linear, rod-like shape.
The simplest member of the alkyne series is ethyne, CtwoH2, commonly called acetylene. The Lewis structure for ethyne, a linear molecule, is:
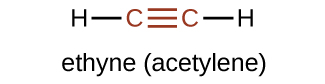
The IUPAC classification for alkynes is similar to that for alkenes except that the suffix -yne is used to indicate a triple bond in the chain. For example, [latex]\text{CH}_3\text{CH}_2\text{C}\;{\equiv}\;\text{CH}[/latex] is called i-butyne.
Case vi
Structure of Alkynes
Depict the geometry and hybridization of the carbon atoms in the following molecule:
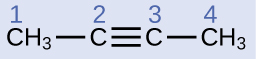
Solution
Carbon atoms 1 and 4 have four single bonds and are thus tetrahedral with sp 3 hybridization. Carbon atoms 2 and iii are involved in the triple bond, so they accept linear geometries and would be classified equally sp hybrids.
Check Your Learning
Identify the hybridization and bail angles at the carbon atoms in the molecule shown:
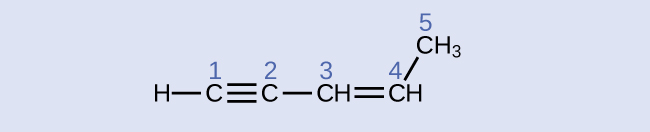
Answer:
carbon ane: sp, 180°; carbon 2: sp, 180°; carbon three: sp 2, 120°; carbon 4: sp 2, 120°; carbon five: sp 3, 109.5°
Chemically, the alkynes are similar to the alkenes. Since the [latex]\text{C}\;{\equiv}\;\text{C}[/latex] functional group has two π bonds, alkynes typically react fifty-fifty more readily, and react with twice as much reagent in add-on reactions. The reaction of acetylene with bromine is a typical instance:

Acetylene and the other alkynes likewise burn readily. An acetylene torch takes reward of the high heat of combustion for acetylene.
Aromatic Hydrocarbons
Benzene, C6H6, is the simplest fellow member of a large family unit of hydrocarbons, called aromatic hydrocarbons. These compounds contain ring structures and exhibit bonding that must be described using the resonance hybrid concept of valence bond theory or the delocalization concept of molecular orbital theory. (To review these concepts, refer to the earlier chapters on chemical bonding). The resonance structures for benzene, Chalf-dozenH6, are:
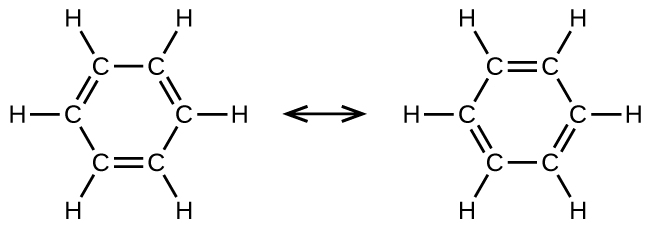
Valence bond theory describes the benzene molecule and other planar aromatic hydrocarbon molecules as hexagonal rings of sp 2-hybridized carbon atoms with the unhybridized p orbital of each carbon atom perpendicular to the plane of the ring. Three valence electrons in the sp 2 hybrid orbitals of each carbon atom and the valence electron of each hydrogen atom grade the framework of σ bonds in the benzene molecule. The quaternary valence electron of each carbon cantlet is shared with an side by side carbon atom in their unhybridized p orbitals to yield the π bonds. Benzene does non, however, showroom the characteristics typical of an alkene. Each of the six bonds betwixt its carbon atoms is equivalent and exhibits properties that are intermediate betwixt those of a C–C single bond and a [latex]\text{C}\;=\;\text{C}[/latex] double bond. To stand for this unique bonding, structural formulas for benzene and its derivatives are typically drawn with unmarried bonds between the carbon atoms and a circle within the band every bit shown in Figure 10.
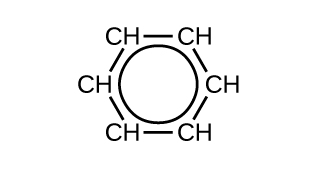
There are many derivatives of benzene. The hydrogen atoms can be replaced past many dissimilar substituents. Aromatic compounds more readily undergo substitution reactions than addition reactions; replacement of one of the hydrogen atoms with another substituent volition leave the delocalized double bonds intact. The following are typical examples of substituted benzene derivatives:
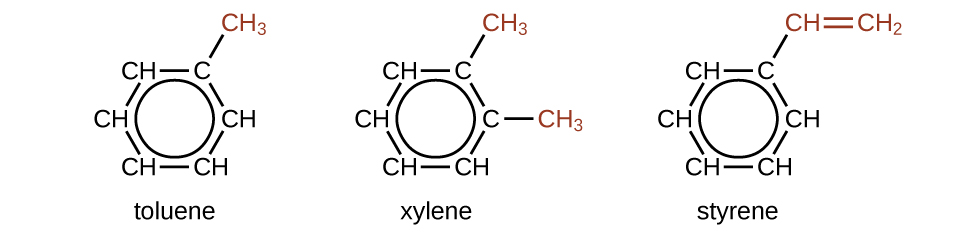
Toluene and xylene are of import solvents and raw materials in the chemical industry. Styrene is used to produce the polymer polystyrene.
Case seven
Structure of Aromatic Hydrocarbons
One possible isomer created by a substitution reaction that replaces a hydrogen atom attached to the effluvious ring of toluene with a chlorine atom is shown hither. Describe two other possible isomers in which the chlorine atom replaces a dissimilar hydrogen atom attached to the aromatic ring:
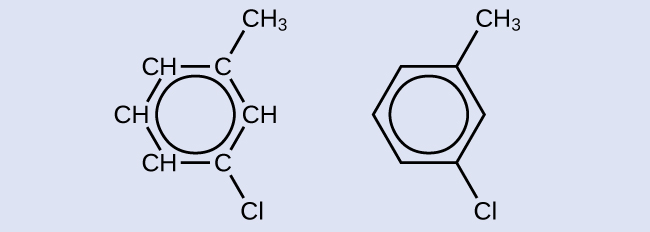
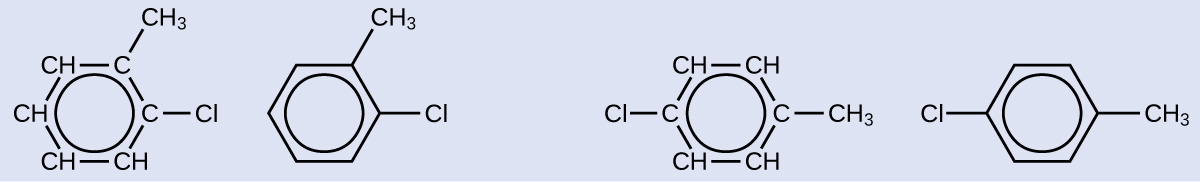
Solution
Since the six-carbon ring with alternate double bonds is necessary for the molecule to be classified as aromatic, appropriate isomers can exist produced only past changing the positions of the chloro-substituent relative to the methyl-substituent:
Check Your Learning
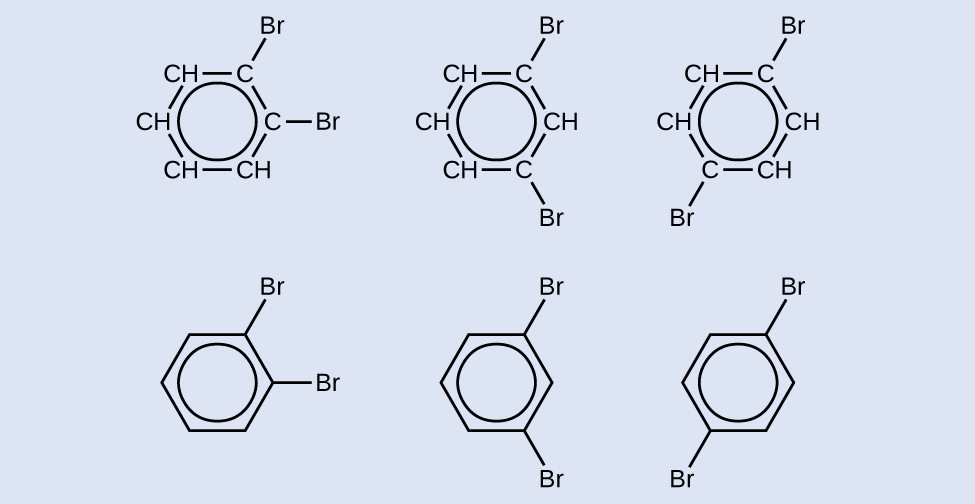
Depict three isomers of a six-membered aromatic band compound substituted with two bromines.
Answer:
Key Concepts and Summary
Strong, stable bonds betwixt carbon atoms produce complex molecules containing bondage, branches, and rings. The chemistry of these compounds is called organic chemistry. Hydrocarbons are organic compounds equanimous of but carbon and hydrogen. The alkanes are saturated hydrocarbons—that is, hydrocarbons that comprise but unmarried bonds. Alkenes incorporate one or more carbon-carbon double bonds. Alkynes contain one or more carbon-carbon triple bonds. Aromatic hydrocarbons incorporate ring structures with delocalized π electron systems.
Chemistry End of Chapter Exercises
- Write the chemical formula and Lewis construction of the following, each of which contains five carbon atoms:
(a) an alkane
(b) an alkene
(c) an alkyne
- What is the divergence between the hybridization of carbon atoms' valence orbitals in saturated and unsaturated hydrocarbons?
- On a microscopic level, how does the reaction of bromine with a saturated hydrocarbon differ from its reaction with an unsaturated hydrocarbon? How are they similar?
- On a microscopic level, how does the reaction of bromine with an alkene differ from its reaction with an alkyne? How are they similar?
- Explicate why unbranched alkenes tin form geometric isomers while unbranched alkanes cannot. Does this explanation involve the macroscopic domain or the microscopic domain?
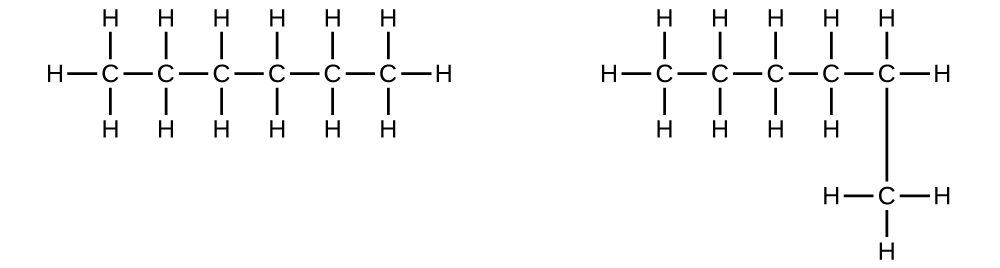
- Explain why these two molecules are not isomers:

- Explain why these two molecules are non isomers:
- How does the carbon-atom hybridization change when polyethylene is prepared from ethylene?
- Write the Lewis structure and molecular formula for each of the following hydrocarbons:
(a) hexane
(b) iii-methylpentane
(c) cis-iii-hexene
(d) 4-methyl-ane-pentene
(e) three-hexyne
(f) 4-methyl-ii-pentyne
- Write the chemical formula, condensed formula, and Lewis construction for each of the following hydrocarbons:
(a) heptane
(b) three-methylhexane
(c) trans-3-heptene
(d) iv-methyl-ane-hexene
(e) 2-heptyne
(f) 3,4-dimethyl-ane-pentyne
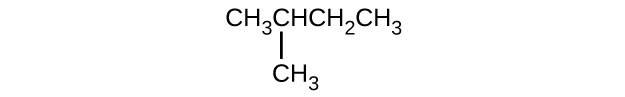
- Give the consummate IUPAC proper noun for each of the following compounds:
(a) [latex]\text{CH}_3\text{CH}_2\text{CBr}_2\text{CH}_3[/latex]
(b) [latex](\text{CH}_3)_3\text{CCl}[/latex]
(c)
(d) [latex]\text{CH}_3\text{CH}_2\text{C}\;{\equiv}\;\text{CH\;CH}_3\text{CH}_2\text{C}\;{\equiv}\;\text{CH}[/latex]
(eastward)
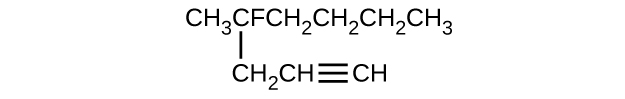
(f)
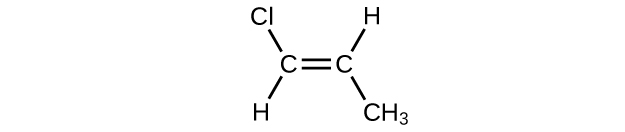
(g) [latex](\text{CH}_3)_2\text{CHCH}_2\text{CH} = \text{CH}_2[/latex]
- Give the complete IUPAC proper noun for each of the following compounds:
(a) [latex](\text{CH}_3)_2\text{CHF}[/latex]
(b) [latex]\text{CH}_3\text{CHClCHClCH}_3[/latex]
(c)
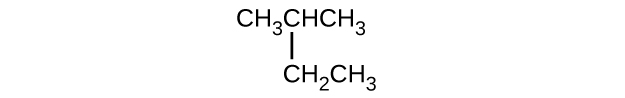
(d) [latex]\text{CH}_3\text{CH}_2\text{CH} = \text{CHCH}_3[/latex]
(e)
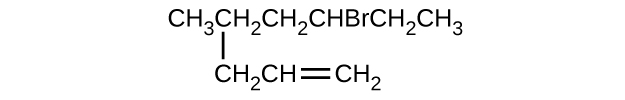
(f) [latex](\text{CH}_3)_3\text{CCH}_2\text{C}{\equiv}\text{CH}[/latex]
- Butane is used every bit a fuel in disposable lighters. Write the Lewis structure for each isomer of butane.
- Write Lewis structures and name the 5 structural isomers of hexane.
- Write Lewis structures for the cis–trans isomers of [latex]\text{CH}_3\text{CH} = \text{CHCl}[/latex].
- Write structures for the three isomers of the aromatic hydrocarbon xylene, [latex]\text{C}_6\text{H}_4(\text{CH}_3)_2[/latex].
- Isooctane is the common name of the isomer of [latex]\text{C}_8\text{H}_18[/latex] used as the standard of 100 for the gasoline octane rating:
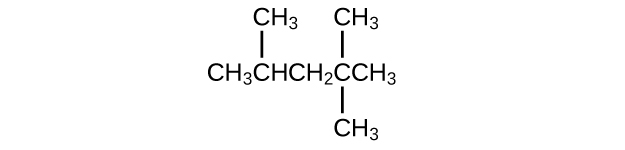
(a) What is the IUPAC name for the compound?
(b) Name the other isomers that incorporate a five-carbon concatenation with three methyl substituents.
- Write Lewis structures and IUPAC names for the alkyne isomers of [latex]\text{C}_4\text{H}_6[/latex].
- Write Lewis structures and IUPAC names for all isomers of [latex]\text{C}_4\text{H}_9\text{Cl}[/latex].
- Proper noun and write the structures of all isomers of the propyl and butyl alkyl groups.
- Write the structures for all the isomers of the [latex]-\text{C}_5\text{H}_{11}[/latex] alkyl grouping.
- Write Lewis structures and describe the molecular geometry at each carbon cantlet in the following compounds:
(a) cis-iii-hexene
(b) cis-1-chloro-2-bromoethene
(c) two-pentyne
(d) trans–6-ethyl-7-methyl-2-octene
- Benzene is ane of the compounds used as an octane enhancer in unleaded gasoline. It is manufactured by the catalytic conversion of acetylene to benzene:[latex]3\text{C}_2\text{H}_2\;{\longrightarrow}\;\text{C}_6\text{H}_6[/latex]
Depict Lewis structures for these compounds, with resonance structures every bit appropriate, and make up one's mind the hybridization of the carbon atoms in each.
- Teflon is prepared by the polymerization of tetrafluoroethylene. Write the equation that describes the polymerization using Lewis symbols.
- Write ii complete, balanced equations for each of the following reactions, i using condensed formulas and one using Lewis structures.
(a) ane mol of one-butyne reacts with 2 mol of iodine.
(b) Pentane is burned in air.
- Write 2 consummate, balanced equations for each of the following reactions, ane using condensed formulas and ane using Lewis structures.
(a) 2-butene reacts with chlorine.
(b) benzene burns in air.
- What mass of 2-bromopropane could be prepared from 25.5 m of propene? Assume a 100% yield of product.
- Acetylene is a very weak acid; however, it volition react with moist silver(I) oxide and form water and a chemical compound equanimous of silver and carbon. Improver of a solution of HCl to a 0.2352-chiliad sample of the compound of silverish and carbon produced acetylene and 0.2822 g of AgCl.
(a) What is the empirical formula of the compound of silver and carbon?
(b) The product of acetylene on improver of HCl to the compound of silver and carbon suggests that the carbon is present as the acetylide ion, [latex]\text{C}_2^{\;\;2-}[/latex]. Write the formula of the chemical compound showing the acetylide ion.
- Ethylene can be produced by the pyrolysis of ethane:[latex]\text{C}_2\text{H}_6\;{\longrightarrow}\;\text{C}_2\text{H}_4\;+\;\text{H}_2[/latex]
How many kilograms of ethylene is produced by the pyrolysis of 1.000 × 103 kg of ethane, bold a 100.0% yield?
Glossary
- addition reaction
- reaction in which a double carbon-carbon bail forms a single carbon-carbon bail by the addition of a reactant. Typical reaction for an alkene.
- alkane
- molecule consisting of only carbon and hydrogen atoms continued by single (σ) bonds
- alkene
- molecule consisting of carbon and hydrogen containing at least one carbon-carbon double bond
- alkyl group
- substituent, consisting of an alkane missing i hydrogen atom, fastened to a larger structure
- alkyne
- molecule consisting of carbon and hydrogen containing at least one carbon-carbon triple bond
- effluvious hydrocarbon
- circadian molecule consisting of carbon and hydrogen with delocalized alternate carbon-carbon single and double bonds, resulting in enhanced stability
- functional group
- part of an organic molecule that imparts a specific chemic reactivity to the molecule
- organic chemical compound
- natural or constructed compound that contains carbon
- saturated hydrocarbon
- molecule containing carbon and hydrogen that has only unmarried bonds between carbon atoms
- skeletal structure
- autograph method of cartoon organic molecules in which carbon atoms are represented past the ends of lines and bends in between lines, and hydrogen atoms attached to the carbon atoms are non shown (but are understood to exist present past the context of the construction)
- substituent
- branch or functional group that replaces hydrogen atoms in a larger hydrocarbon concatenation
- commutation reaction
- reaction in which i atom replaces some other in a molecule
Solutions
Answers to Chemistry Terminate of Affiliate Exercises
1. At that place are several sets of answers; ane is:
(a) [latex]\text{C}_5\text{H}_{12}[/latex]
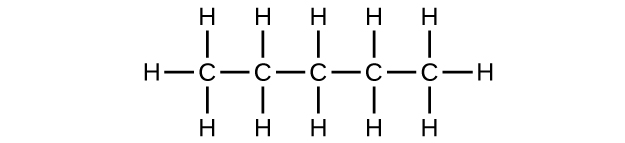 ;
;
(b) [latex]\text{C}_5\text{H}_{10}[/latex]
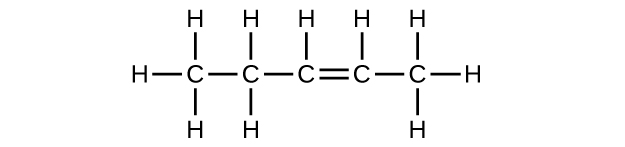 ;
;
(c) [latex]\text{C}_5\text{H}_8[/latex]
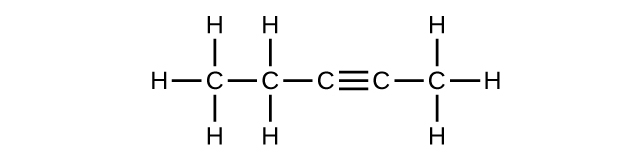
3. Both reactions upshot in bromine being incorporated into the construction of the product. The departure is the mode in which that incorporation takes place. In the saturated hydrocarbon, an existing C–H bond is broken, and a bond betwixt the C and the Br can then be formed. In the unsaturated hydrocarbon, the only bond broken in the hydrocarbon is the π bail whose electrons can be used to form a bond to one of the bromine atoms in Br2 (the electrons from the Br–Br bail form the other C–Br bond on the other carbon that was office of the π bond in the starting unsaturated hydrocarbon).
v. Unbranched alkanes have gratis rotation about the C–C bonds, yielding all orientations of the substituents about these bonds equivalent, interchangeable past rotation. In the unbranched alkenes, the inability to rotate about the [latex]\text{C}\;=\;\text{C}[/latex] bail results in stock-still (unchanging) substituent orientations, thus permitting unlike isomers. Since these concepts pertain to phenomena at the molecular level, this caption involves the microscopic domain.
7. They are the same chemical compound because each is a saturated hydrocarbon containing an unbranched chain of six carbon atoms.
9. (a) [latex]\text{C}_6\text{H}_{14}[/latex]
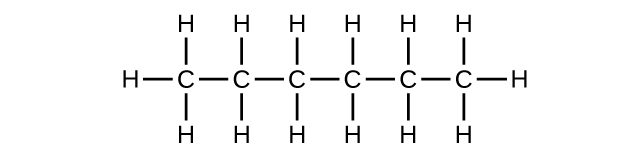 ;
;
(b) [latex]\text{C}_6\text{H}_{14}[/latex]
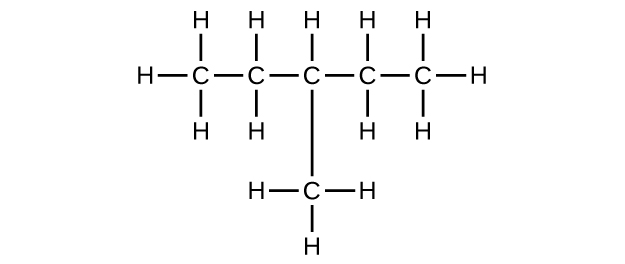 ;
;
(c) [latex]\text{C}_6\text{H}_{12}[/latex]
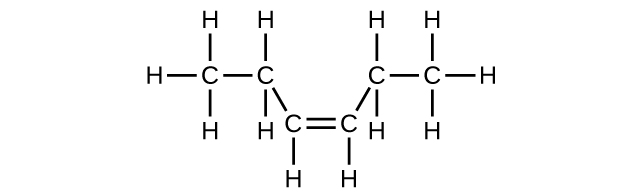 ;
;
(d) [latex]\text{C}_6\text{H}_{12}[/latex]
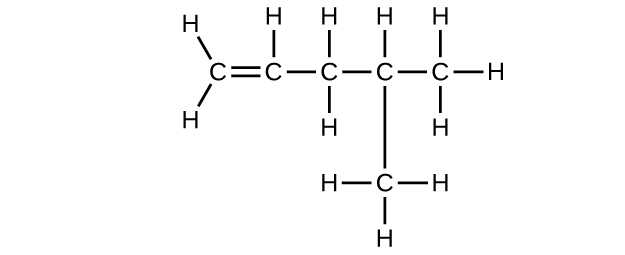 ;
;
(e) [latex]\text{C}_6\text{H}_{10}[/latex]
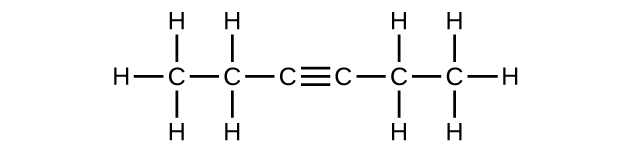 ;
;
(f) [latex]\text{C}_6\text{H}_{10}[/latex]
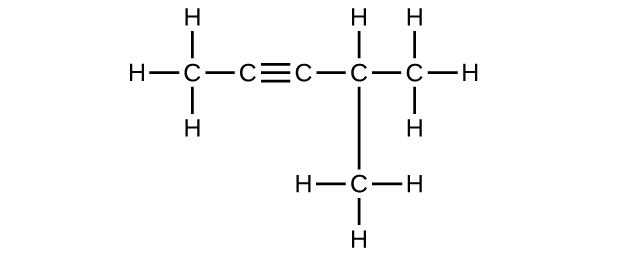
11. (a) 2,two-dibromobutane; (b) 2-chloro-2-methylpropane; (c) 2-methylbutane; (d) 1-butyne; (e) iv-fluoro-4-methyl-1-octyne; (f) trans-1-chloropropene; (grand) 5-methyl-1-pentene
thirteen.
15.
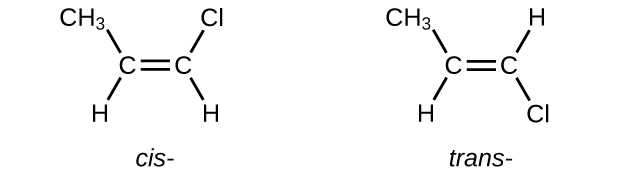
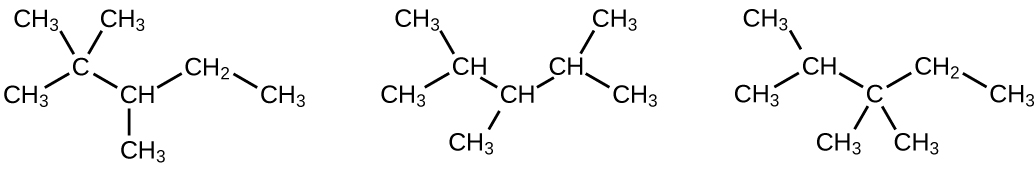
17. (a) 2,2,4-trimethylpentane; (b) ii,2,3-trimethylpentane, 2,iii,four-trimethylpentane, and 2,three,three-trimethylpentane:
19.
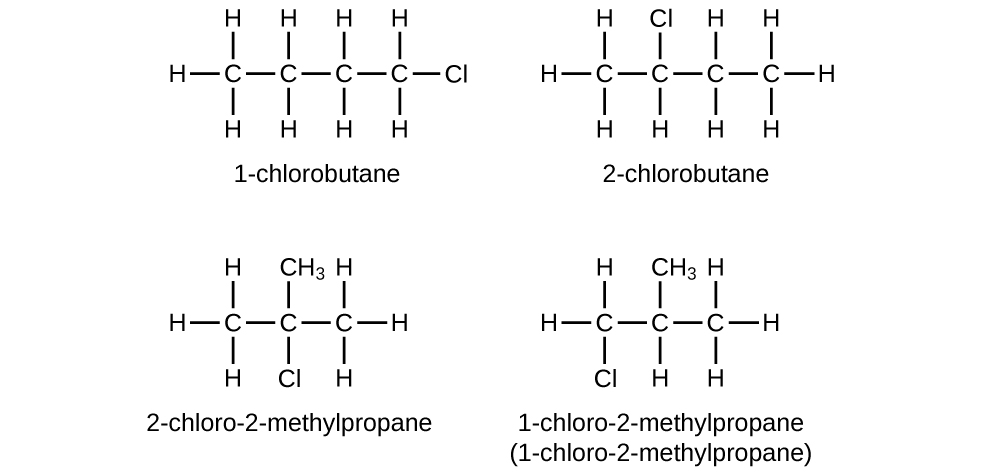
21. In the following, the carbon backbone and the advisable number of hydrogen atoms are shown in condensed form:
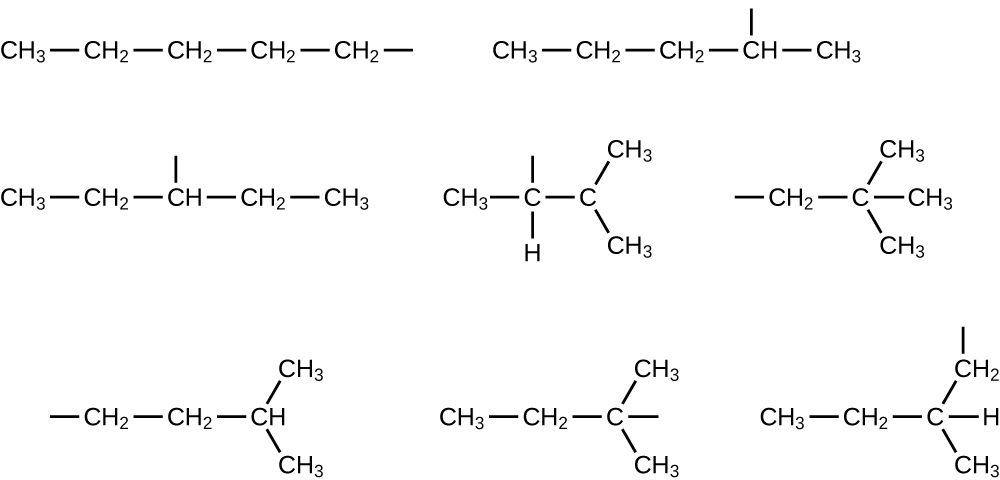
23.
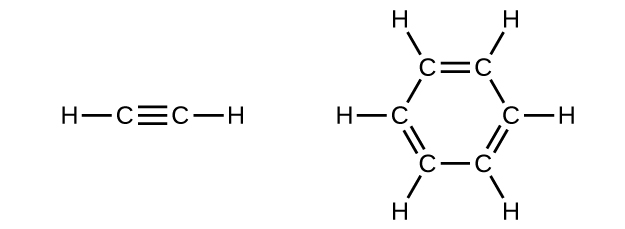
In acetylene, the bonding uses sp hybrids on carbon atoms and s orbitals on hydrogen atoms. In benzene, the carbon atoms are sp 2 hybridized.
25. (a) [latex]\text{CH}\;{\equiv}\;\text{CCH}_2\text{CH}_3\;+\;2\text{I}_2\;{\longrightarrow}\;\text{CHI}_2\text{CI}_2\text{CH}_2\text{CH}_3[/latex]
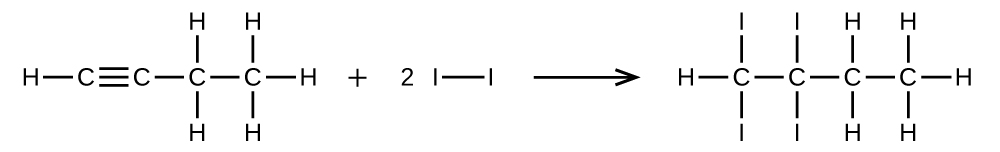
(b) [latex]\text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_2\text{CH}_3\;+\;8\text{O}_2\;{\longrightarrow}\;v\text{CO}_2\;+\;half dozen\text{H}_2\text{O}[/latex]
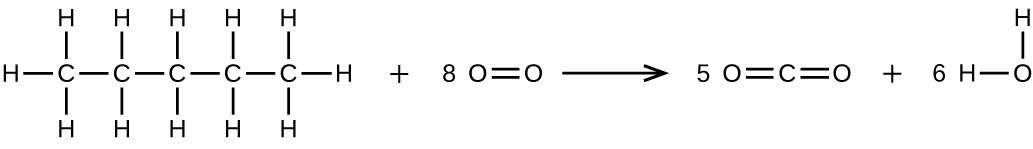
27. 65.ii k
29. nine.328 × 102 kg
Source: https://opentextbc.ca/chemistry/chapter/20-1-hydrocarbons/
0 Response to "Which Eas Reaction Can Be Used to Make a Carbon-carbon Bond in the Synthesis of Diazepam"
Post a Comment